Mesenchymal Stem Cell Review 2025: Latest Research, Uses and Future Directions
Mesenchymal stem cells (MSCs) have emerged as one of the most promising tools in modern regenerative medicine due to their unique ability to differentiate into various cell types and modulate immune responses. Sourced from tissues like bone marrow, adipose fat, and umbilical cords, MSCs have been studied extensively for their role in treating orthopedic injuries, autoimmune conditions, and even viral infections such as COVID-19. This mesenchymal stem cell review explores the latest scientific findings, therapeutic applications, and future directions of MSC-based therapies—highlighting both their clinical potential and the challenges that still lie ahead.
What Are Mesenchymal Stem Cells (MSCs)?
Mesenchymal stem cells (MSCs) are multipotent adult stem cells capable of self-renewal and differentiation into various mesodermal cell types, including bone, cartilage, and fat. Characterized by specific surface markers (such as CD73, CD90, and CD105), MSCs are non-hematopoietic and adhere to plastic under standard lab conditions. They can be sourced from bone marrow, adipose tissue, umbilical cord, and dental pulp—each offering unique advantages in yield, proliferation, and clinical usability.
Unlike embryonic stem cells, which are pluripotent, MSCs have limited differentiation potential but offer fewer ethical concerns and a lower risk of tumor formation. Compared to hematopoietic stem cells, MSCs play a more prominent role in connective tissue regeneration and immune regulation. Due to these properties, MSCs are widely explored in regenerative medicine and cell therapy, particularly for orthopedic repair, autoimmune diseases, and inflammation-related conditions.
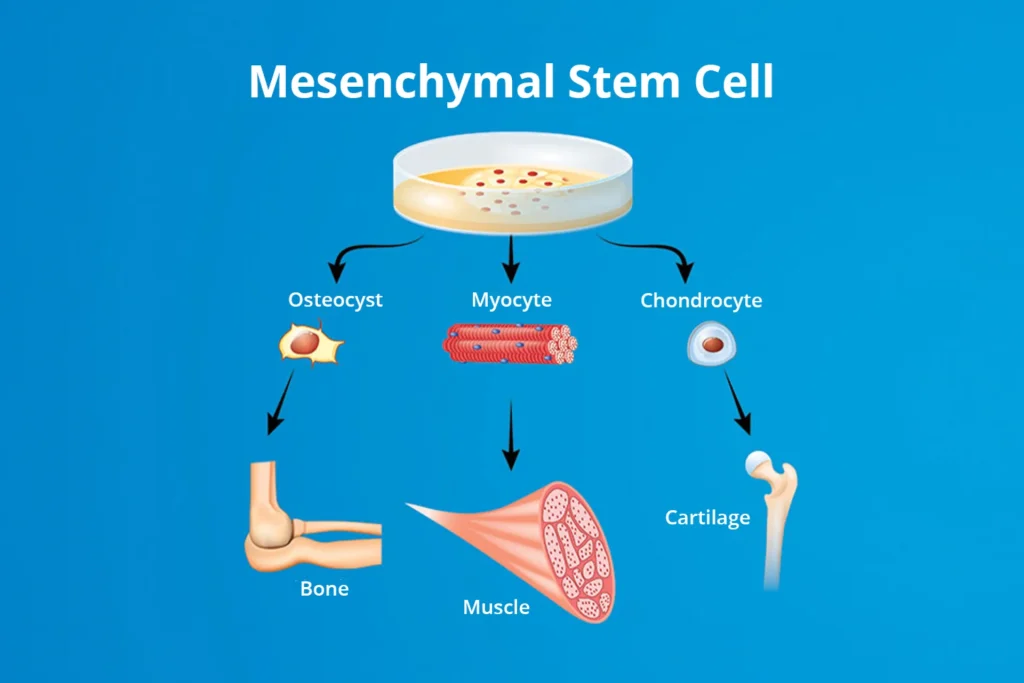
Trilineage Differentiation Explained
MSCs are best known for their ability to undergo trilineage differentiation, meaning they can become three primary cell types:
These lineages reflect the core of mesenchymal stem cell lineage research and are considered a standard benchmark for confirming MSC identity in vitro.
Key Transcription Factors and Markers
Lineage commitment is guided by specific transcription factors:
These markers are commonly used in differentiation assays to verify successful MSC conversion into target cell types.
In Vitro vs In Vivo Differentiation
In vitro settings, MSCs are exposed to controlled media and supplements that direct lineage commitment. This enables researchers to test MSC potential and behavior under predictable conditions. However, in vivo applications, MSC differentiation is far more complex—heavily influenced by the local tissue environment, extracellular matrix, and signaling molecules.
Impact on Tissue Repair and Regenerative Therapies
The ability of MSCs to regenerate bone, cartilage, and soft tissues makes them highly attractive for wound healing, orthopedic treatments, and reconstructive surgery. Their stem cell plasticity enables them to adapt and respond to various physiological needs, contributing to tissue remodeling and functional recovery.
This dynamic nature of MSC differentiation pathways forms the backbone of many current and future cell-based therapies.
Immunomodulatory and Anti-Inflammatory Effects of MSCs
Beyond their regenerative potential, mesenchymal stem cells (MSCs) exhibit powerful immunomodulatory and anti-inflammatory effects, making them valuable in treating immune-related and inflammatory disorders. Their ability to interact with and regulate the immune system distinguishes them from many other stem cell types.
Interaction with Immune Cells
MSCs influence both the innate and adaptive immune systems. They modulate the activity of:
These interactions play a critical role in immune regulation and controlling overactive immune responses.
MSC Therapy in Autoimmune Diseases
Due to their immune-modulating properties, MSCs are being explored in the treatment of various autoimmune disorders such as:
Clinical trials are ongoing to validate their safety and efficacy in these areas.
Cytokine Secretion and Immune Signaling
MSCs exert their anti-inflammatory effects through paracrine signaling, releasing a wide array of bioactive molecules such as:
This cytokine secretion supports tissue regeneration while dampening harmful inflammation.
Toward Off-the-Shelf Cell Therapies
Thanks to their low immunogenicity and ability to avoid immune rejection, MSCs are considered ideal for allogeneic (donor-derived) or “off-the-shelf” stem cell therapy. This opens the door to scalable, ready-to-use cell-based treatments for inflammatory and immune-mediated conditions
With their dual role in tissue regeneration and immune modulation, MSCs continue to gain traction as a powerful therapeutic platform in both acute and chronic inflammatory diseases.
Clinical Applications and Therapeutic Potential
Mesenchymal stem cells (MSCs) have transitioned from experimental models to clinical settings, demonstrating promising results in treating a wide range of conditions. Their regenerative, anti-inflammatory, and immunomodulatory properties have positioned them at the forefront of modern stem cell therapy and regenerative treatment strategies.
Tumorigenicity and Long-Term Safety
Although MSCs are considered relatively safe compared to embryonic or induced pluripotent stem cells, concerns remain about their tumorigenic potential, especially after extensive expansion or genetic modification. Long-term data on safety, especially for repeated or high-dose applications, are still limited. Regulatory agencies like the FDA and EMA emphasize the need for rigorous preclinical testing and long-term follow-up in clinical trials.
Global Regulatory Landscape
The regulatory environment for MSC therapies varies widely across countries. In the United States, the FDA classifies MSC-based products as biologics, requiring full Investigational New Drug (IND) applications. The European Medicines Agency (EMA) has similar oversight under Advanced Therapy Medicinal Products (ATMP) regulations. Many MSC therapies remain unapproved or are limited to compassionate use, while others operate in legal gray areas or under looser frameworks in certain countries.
These regulatory and biological limitations must be addressed through standardized protocols, international collaboration, and transparent reporting if MSCs are to become a fully integrated part of evidence-based medicine.
Limitations and Regulatory Challenges
While mesenchymal stem cells (MSCs) hold enormous promise in regenerative medicine, their clinical translation is not without challenges. Issues related to consistency, safety, and global regulation continue to affect the widespread adoption of MSC-based therapies.
Donor Variability and Cellular Aging
One of the major limitations in MSC therapy is donor-to-donor variability. MSCs sourced from different individuals or tissues (e.g., bone marrow vs. adipose tissue) can vary significantly in their proliferation rate, differentiation capacity, and immunomodulatory effects. Additionally, cellular aging during in vitro expansion reduces their therapeutic efficacy and may introduce unwanted changes such as senescence or reduced plasticity, which compromises clinical outcomes.
Standardization and Quality Control
Despite growing clinical use, standardization in MSC manufacturing remains a critical hurdle. Variations in isolation techniques, culture conditions, and passage numbers lead to inconsistencies in cell quality. To ensure therapeutic reliability, strict MSC quality control and adherence to GMP (Good Manufacturing Practice) compliance are essential, yet difficult to maintain across global laboratories and production centers.
Future Trends in MSC Research and Development
As regenerative medicine continues to evolve, mesenchymal stem cell (MSC) research is moving beyond traditional applications into more innovative, scalable, and targeted therapies. Emerging technologies like exosome therapy, gene editing, and bioprinting are reshaping the future of MSC-based treatments.
Exosome-Based Therapies (Cell-Free Regenerative Medicine)
One of the most exciting developments is the use of MSC-derived exosomes—small extracellular vesicles that carry proteins, RNAs, and growth factors. These cell-free therapeutics mimic many of the beneficial effects of MSCs without the risks associated with live cell transplantation. Exosomes are easier to store, scale, and deliver, making them ideal for applications in wound healing, neurodegeneration, and even cosmetic treatments.
Genetically Engineered MSCs (e.g., CRISPR-Modified Cells)
CRISPR-Cas9 technology is enabling the development of genetically enhanced MSCs with improved survival, targeting ability, and therapeutic output. For example, MSCs can be engineered to overexpress anti-inflammatory cytokines or survival factors, increasing their efficacy in chronic disease environments. These CRISPR-MSCs are opening new possibilities in precision medicine and targeted drug delivery.
AI and Biomaterials in Personalized Therapy
Artificial intelligence (AI) is increasingly used to optimize MSC manufacturing, predict differentiation outcomes, and tailor therapy to individual patients. Combined with smart biomaterials, such as responsive hydrogels and bioactive scaffolds, researchers can now create highly controlled microenvironments that direct MSC behavior more precisely.
MSCs and 3D Bioprinting for Tissue Engineering
3D bioprinting enables the construction of tissue-like structures using MSCs and bio-inks. This approach is particularly promising for creating bone, cartilage, and skin grafts for reconstructive surgery. By integrating bioprinting with scaffold technologies and real-time imaging, scientists can fabricate complex, functional tissues customized for each patient.
Together, these trends represent a shift from conventional MSC therapy to next-generation regenerative solutions—where cells, biomolecules, and intelligent systems converge to create safer, more effective treatments for complex diseases.